減脂增肌新靶點(diǎn)-ActRII
發(fā)布時(shí)間:2025/10/24分類(lèi):技術(shù)文章來(lái)源:科佰生物
背景
激活素 A(Activin A)是轉(zhuǎn)化生長(zhǎng)因子-β(TGF-β)超家族的重要成員,是參與過(guò)敏、自身免疫性疾病、癌癥和其他免疫紊亂疾病的多能因子。ACVR2A(激活素受體2A)和ACVR2B(激活素受體2B)作為Activin A的II型受體,它們?cè)诩?xì)胞生長(zhǎng)、分化和組織修復(fù)等多種生理過(guò)程中發(fā)揮關(guān)鍵作用。這兩種跨膜蛋白具有富含半胱氨酸的配體結(jié)合域和激酶活性催化結(jié)構(gòu)域,通過(guò)與激活素等配體結(jié)合,激活下游信號(hào)通路如Smad2/3和MAPK,從而調(diào)節(jié)骨骼肌質(zhì)量、脂肪代謝、胚胎發(fā)育等。由于其在肌肉和脂肪代謝中的重要性,ACVR2A和ACVR2B已成為治療肌肉萎縮和代謝性疾病的潛在藥物靶點(diǎn)。
ActRII的結(jié)構(gòu)與功能
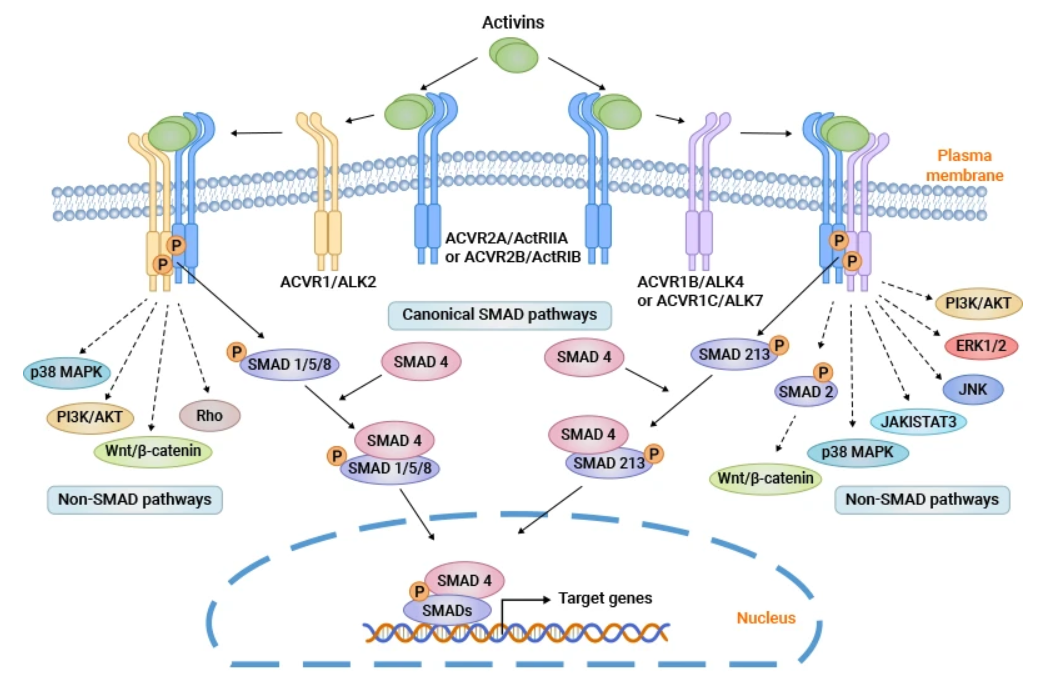
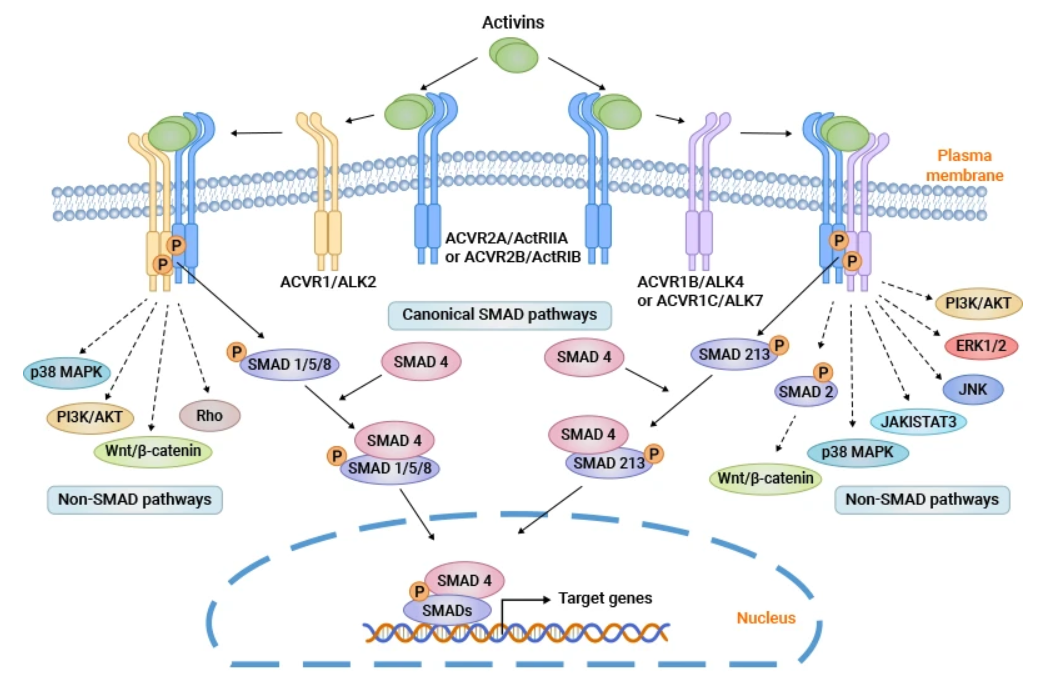
Activin A是一種約25 kDa的細(xì)胞因子,可通過(guò)二硫鍵與βA和/或 βB 亞基連接形成同源二聚體或異二聚體。Activin A通過(guò)兩個(gè)I型和兩個(gè)II型受體發(fā)出信號(hào),配體結(jié)合后,組裝最終的受體復(fù)合物。I型受體包括激活素受體1A型(或激活素受體樣激酶2,ALK2)、激活素受體1B型(或ALK4)和激活素受體1C型(或ALK7)。II型受體(ActRII)分為激活素受體IIA型(ACVR2A)和激活素受體IIB型(ACVR2B)。
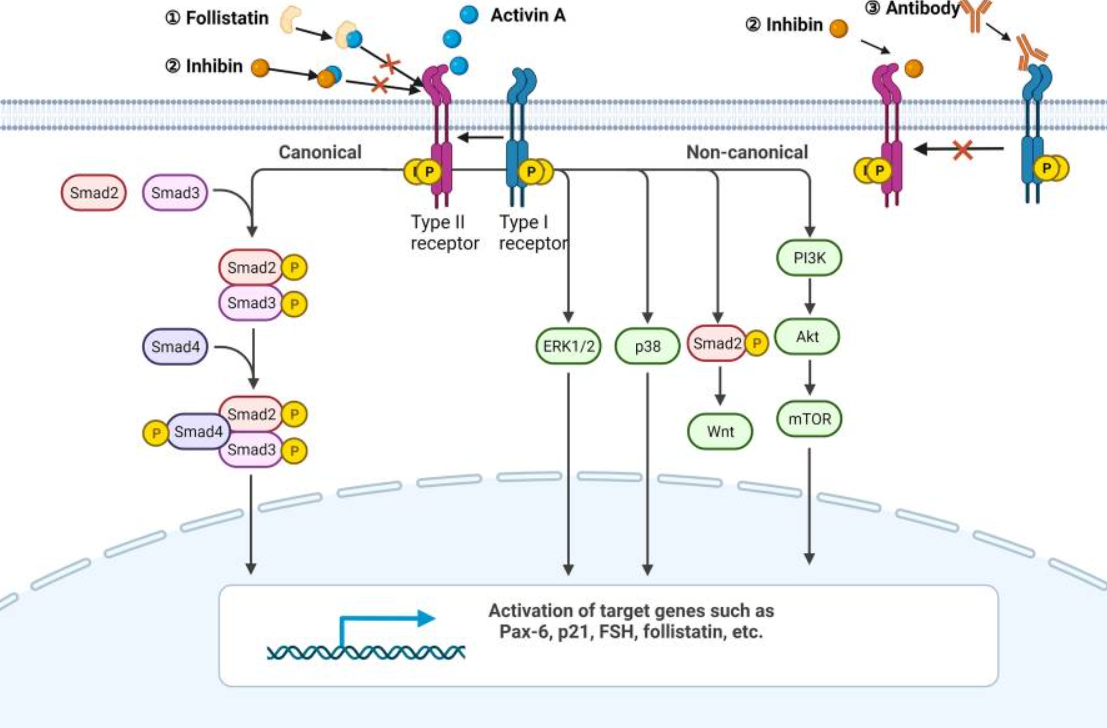
激活素A首先結(jié)合并促進(jìn)ActRII的磷酸化,然后招募I型受體形成磷酸化多聚體。激活的受體復(fù)合物磷酸化SMAD2 和 SMAD3蛋白,磷酸化后的smad2/3與smad4蛋白結(jié)合成三聚體并易位到細(xì)胞核中調(diào)控相關(guān)基因的轉(zhuǎn)錄。
ActRⅡ(激活素Ⅱ型受體(Activin type Ⅱ Receptor))存在于脂肪和肌肉細(xì)胞中。在脂肪細(xì)胞中,激活素通過(guò)ActRⅡ進(jìn)行脂質(zhì)存儲(chǔ),阻斷該信號(hào)通路可促進(jìn)脂肪代謝。在肌肉細(xì)胞中,ActRⅡ受體傳導(dǎo)的信號(hào)通路能夠抑制肌肉生長(zhǎng)并導(dǎo)致其萎縮,阻斷骨骼肌中的激活素信號(hào)可以抑制這種萎縮,并可以促進(jìn)肌肉質(zhì)量的增加,幫助肥胖患者在減肥的同時(shí)改善身體成分和代謝。
靶向ActRⅡ的藥物研發(fā)進(jìn)展
關(guān)于ActRⅡ,常用的靶向療法主要聚焦于融合蛋白和抗體。目前已有兩款藥物上市,為BMS/默沙東的ActRIIB-Fc融合蛋白藥物L(fēng)uspatercept(治療地中海貧血)和默沙東開(kāi)發(fā)的ActRIIA-Fc融合蛋白藥物Sotatercept(治療肺動(dòng)脈高壓)。
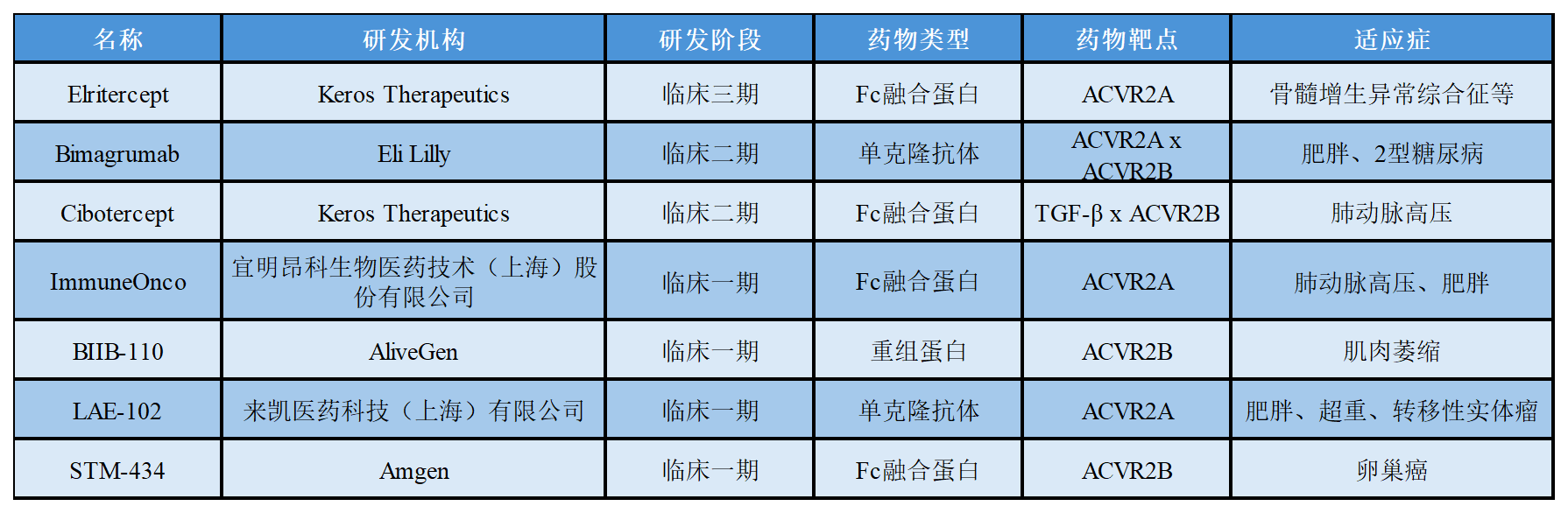
關(guān)于ActRⅡ在研的部分藥物進(jìn)展如下表所示:
ActRⅡ的細(xì)胞模型
為助力ActRⅡ靶點(diǎn)藥物研發(fā),南京科佰開(kāi)發(fā)了Activin A Effector Reporter Cell、ACVR2A CHO、ACVR2A Effector Reporter Cell、ACVR2B CHO和ACVR2B Effector Reporter Cell細(xì)胞篩選模型,部分?jǐn)?shù)據(jù)展示如下:
部分?jǐn)?shù)據(jù)展示
Activin A Effector Reporter Cell CBP74225
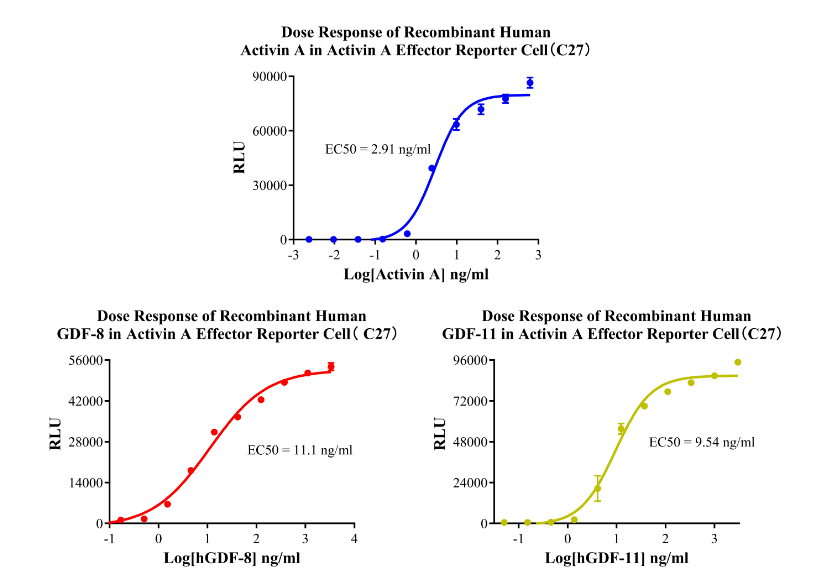
Figure 3.Dose Response of Recombinant Human Activin A in Activin A Effector Reporter Cell( C27).Dose Response of Recombinant Human GDF-8 in Activin A Effector Reporter Cell( C27).Dose Response of Recombinant Human GDF-11 in Activin A Effector Reporter Cell(C27).
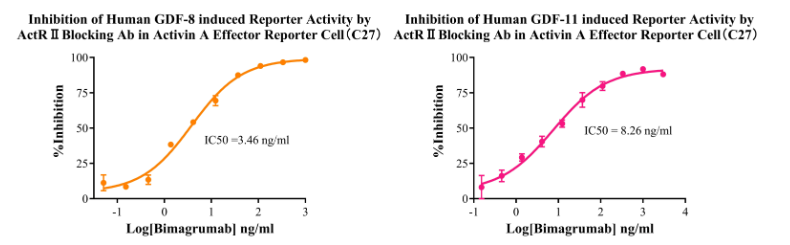
Figure 4. Inhibition of Human GDF-8 induced Reporter Activity by ActR II Blocking Ab in Activin A Effector Reporter Cell(C27). Inhibition of Human GDF-11 induced Reporter Activity by ActR II Blocking Ab in Activin A Effector Reporter Cell(C27).
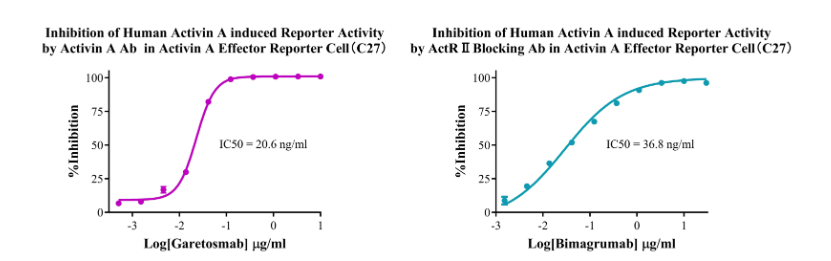
Figure 5. Inhibition of Human Activin A induced Reporter Activity by Activin A Ab in Activin A Effector Reporter Cell(C27). Inhibition of Human Activin A induced Reporter Activity by ActR II Blocking Ab in Activin A Effector Reporter Cell(C27).
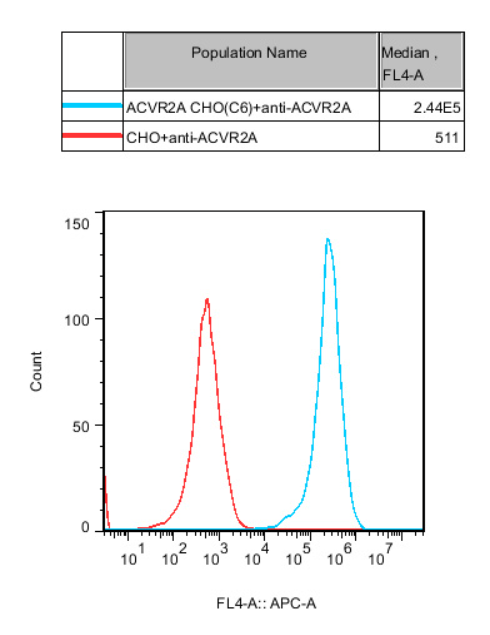
Figure 6. Recombinant ACVR2A CHO stably expressing ACVR2A.
ACVR2A Effector Reporter Cell CBP74277
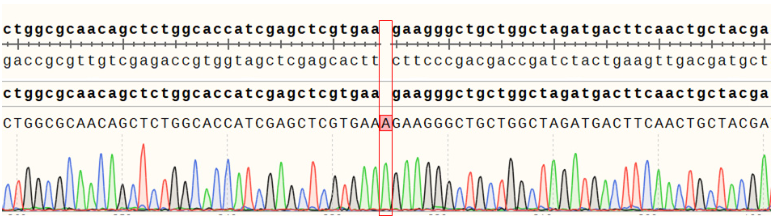
Figure 7.ACVR2A Effector Reporter Cell 測(cè)序結(jié)果 ACVR2B(NM_001106.4): c.221_222insA/ACVR2B:p.K74Kfs*7
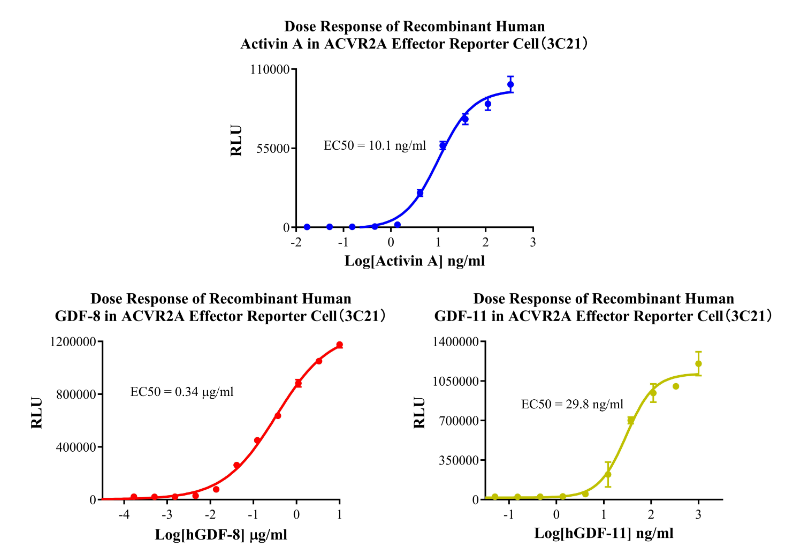
Figure 8. Dose Response of Recombinant Human Activin A in ACVR2A Effector Reporter Cell(3C21).Dose Response of Recombinant Human GDF-8 in ACVR2A Effector Reporter Cell (3C21). Dose Response of Recombinant Human GDF-11 in ACVR2A Effector Reporter Cell (3C21).
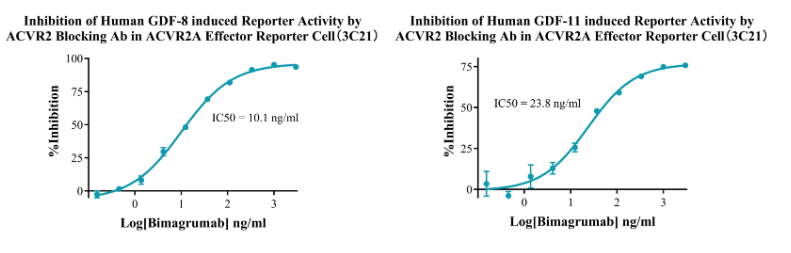
Figure 9. Inhibition of Human GDF-8 induced Reporter Activity by ACVR2 Blocking Ab in ACVR2A Effector Reporter Cell (3C21).Inhibition of Human GDF-11 induced Reporter Activity by ACVR2 Blocking Ab in ACVR2A Effector Reporter Cell (3C21).
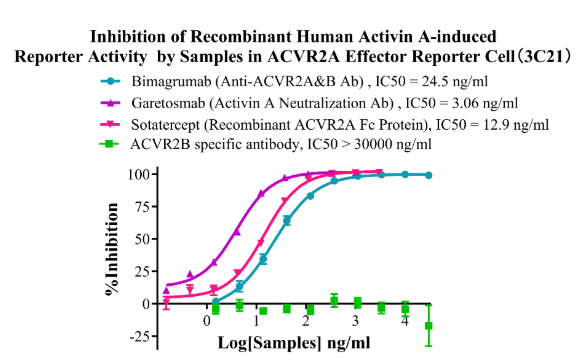
Figure10. Inhibition of Recombinant Human Activin A-induced Reporter Activity by Samples in ACVR2A Effector Reporter Cell (3C21).
ACVR2B CHO CBP74249
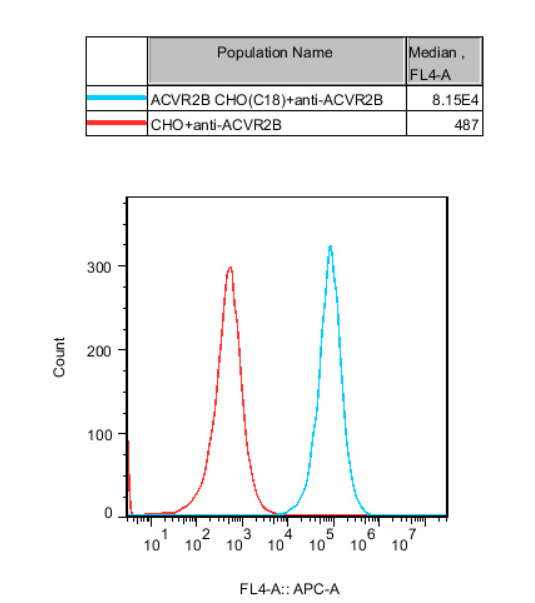
Figure 11.Recombinant ACVR2B CHO stably expressing ACVR2B.
ACVR2B Effector Reporter Cell CBP74280
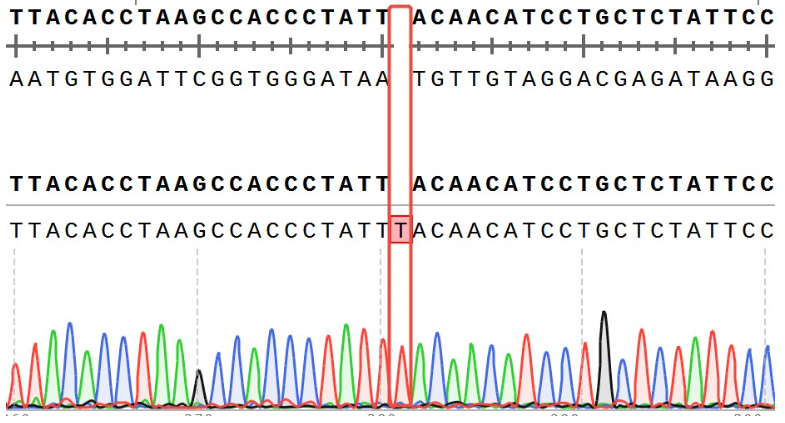
Figure 12. ACVR2B Effector Reporter Cell 測(cè)序結(jié)果 ACVR2A(NM_001616.5): c.409_410insT/ ACVR2A:p.Y137Lfs*76
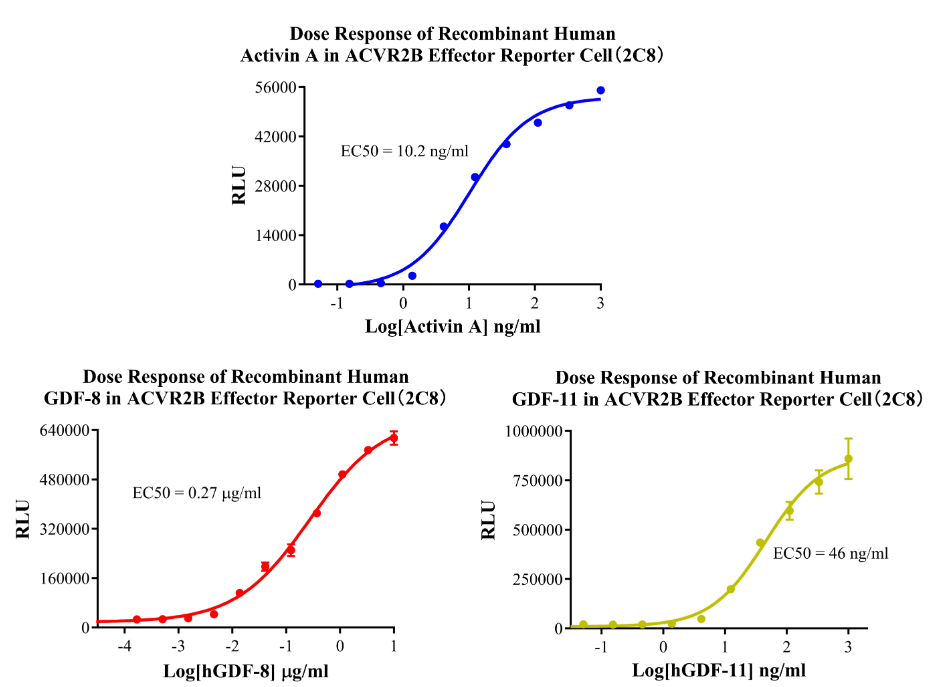
Figure 13.Dose Response of Recombinant Human Activin A in ACVR2B Effector Cell(2C8).Dose Response of Recombinant Human GDF-8 in ACVR2B Effector Reporter Cell(2C8).Dose Response of Recombinant Human GDF-11 in ACVR2B Effector Reporter Cell (2C8).
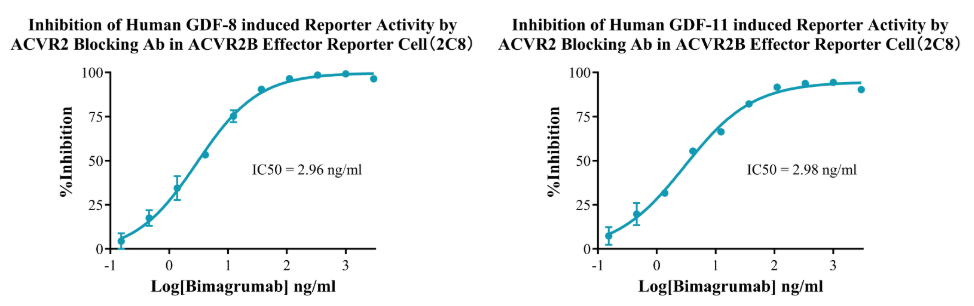
Figure 14. Inhibition of Human GDF-8 induced Reporter Activity byACVR2 Blocking Ab in ACVR2B Effector Reporter Cell(2C8). Inhibition of Human GDF-11 induced Reporter Activity by ACVR2 Blocking Ab in ACVR2B Effector Reporter Cell(2C8).
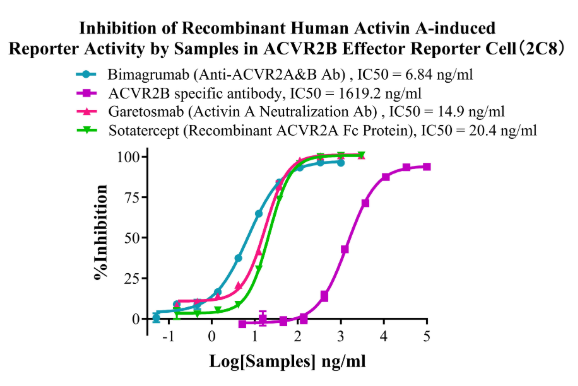
Figure15. Inhibition of Recombinant Human Activin A-induced Reporter Activity by Samples in ACVR2B Effector Reporter Cell (2C8).